Last month, innovators and researchers came together from across the Waikato, Rotorua and the Bay of Plenty to celebrate the prestigious Kudos Science Excellence Awards.
One finalist that shone, placing within the Engineering Science category, was RespiratorNZ and its ventilator initiative.
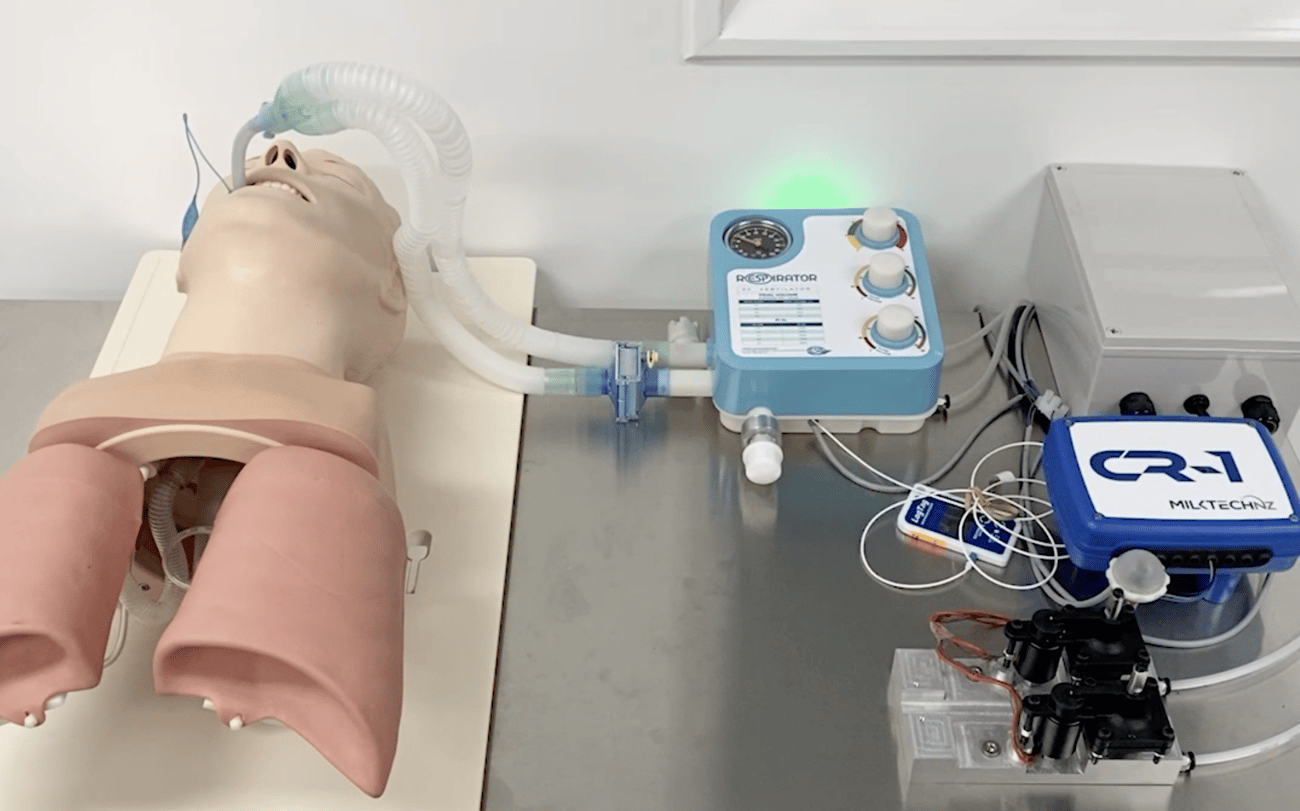
Built by residential neighbours, RespiratorNZ was launched after hearing the country could possibly go into Lockdown, coupled with restricted borders and the potential of overflowing hospital wards lacking necessary equipment like respirators.
With only 58 respirators in New Zealand and no way to bring in more once the borders were shut down, the team took an old respirator from the ED department, pulled it apart and started to engineer components for a Covid respirator.
Idealog chats to co-founder Jeff Sharp on the process of building his business and the potential of exporting across the world.

Who are you?
RespiratorNZ is a branch of ESPlastics Ltd, a 30 year longstanding Waikato based plastics manufacturer.
Where did you get the idea for your project?
The RespitaotrNZ idea was developed days before the nationwide lockdown by myself and my two neighbours who are Emergency Doctors at the Waikato Hospital. The idea came from the growing concern around New Zealand’s capacity to deal with a covid outbreak and the potential that we did not have enough ventilators within the country to deal with the consequences as seen around the world at this time. ESPlastics Ltd has a significant manufacturing capacity, and I was sure there was something we could be doing to ‘ease the load.’
What’s your point of difference or what makes you better than your competitors? Or do you even have any competitors?
Compared to other mechanical ventilators currently in the market, our product was designed with a pandemic environment in mind catering specifically to requirements that become obvious due to the current conditions. We created a simple design, that may be easily picked up by retired medical professionals or trainee doctors and nurses. Our ventilator requires no more than half an hour training before staff should be able to fully operate it. Other features include a light, robust design, with complete mobility, and available at a low cost.
What are the challenges you’ve faced along the way?
The Ventilator was rapidly developed within four weeks, despite this regulatory approvals have been a slow and tedious process, with many countries expressing interest but holding off on buying ventilators until approvals come through.
Sourcing suppliers during lockdown was difficult particularly due to freight being affected by covid nationally and internationally. This made procuring consumables tricky.
What underlying problem does it solve?
The lack of pandemic ventilators and shortfall in ventilator stock due to covid.
What stage is your business at now?
Throughout the lockdown period, ESPlastics ventured into ventilation manufacture, which in turn encouraged us to get full medical device licensing. ESPlastics is now licenced as an ISO 13485 medical device manufacturer giving us the capability to contract manufacture medical products, one of few in NZ. From the whole experience, ESPlastics has been introduced to an entirely new industry that was previously unexplored by the company.
How many stores/sites stocking your technology/product now?
We are currently fielding interest from multiple African countries, the UAE, Indonesia and Canada. This interest is dependant on the pending regulatory approvals from SAHPRA (South Africa), UAE, CE and Health Canada.
What’s next? Where do you hope to take this?
We are currently looking into opens to expand our in-house developed RespiratorNZ product range from just the stand-alone ventilator to a ‘family package’ under our new license. This pack can be deployed in a field hospital setting providing patients with mechanical ventilation without the need for bottles or main supply of air, or mains supply power providing developing countries (where the majority of our interest is coming from) with a comprehensive package to deal with covid and meet mechanical ventilation requirements.
This gives the package complete mobility and transportable properties and is perfect for situations where there is a lack of adequate infrastructure to deal with an influx of patients requiring mechanical ventilation. This may apply to infectious disease outbreaks, natural disaster relief, military or refugee camps and other potential hostile environments.
We are also taking on new customers for contract manufacturing under our new license.
What impact could you envision your product having in the future?
We hope the above-mentioned product package would be able to serve many different regions and scenarios in the future. We still hope to gain regulatory approval for just the ventilator and begin exporting as soon as possible.