Volpara Solutions breast cancer detection technology used on 4 mln women, GE Healthcare tie-up set to ramp up global sales
Having one of the world’s largest medical device companies — GE Healthcare — distribute its technology is set to put Matakina Technology’s breast cancer detection tool, Volpara Solutions, on the bigger world map.
The hard yards put in over the last four years, in putting the technology through numerous trials, is also paying off as the scientific community and positive results are providing validation for the company.
Matakina was founded in 2009 by a group of people, including a physicist and cancer and medical imaging specialist. Matakina’s patented software helps detects dense breast tissues – the higher the density of breast tissues, the more at-risk women are for breast cancer. Matakina, in Maori, means insight, which is what the Volpara software is built to do, providing a close look at breast tissues. Matakina’s products are: VolparaDose, VolparaDensity, VolparaAnalytics, VolparaResearch.
Frost and Sullivan in August 2014 awarded Volpara Solutions with the 2014 North American Frost & Sullivan Award for Technology Innovation Leadership.
Matakina Technology CEO Dr Ralph Highnam says the company is looking to 2016 with confidence, with some big deals being lined up.The company is set to double product revenues in 2015 against last year, which was already 50% higher from 2013’s level.
The company, in December 2014, raised $5.5 million from a group of high net worth individuals and fund managers in Australia and New Zealand, including the Milford Active Growth Fund.
Idealog recently caught up with Dr Highnam who shared some of his thoughts on what the GE Healthcare deal means and the business of setting up a technology business out of NZ.
Q: Matakina Technology Ltd has just signed up an agreement with GE Healthcare to distribute the Volpara Solution screening software/technology. What is the impact of this on your company, and for NZ?
?A: We’ve been picking up significant global traction over the last year with direct sales and local distributor sales and have doubled revenues from product sales. However, GE is one of the biggest, if not the biggest, medical device company in the world and with this deal they will start distributing our products on a scale far beyond what we’ve been able to do to date.
That’s good news for us in that we will now see sales from accounts that we’ve never heard of before, but also introduces new challenges for us in needing to install our products into far away countries where we have no presence. Fortunately, over the last few months we’ve geared the company up to deliver and support globally.
For NZ, this is a major endorsement of the ability to build products here which are truly world-class and market-leading. The engineering and R&D team we have in Wellington builds exceptionally high, FDA (Food and Drug Administration) compliant software products and the R&D team, supported by guests from overseas, is truly world-leading.
Q: What is the volumetric breast density measurement software? What does it do? And how is it different from existing softwares?
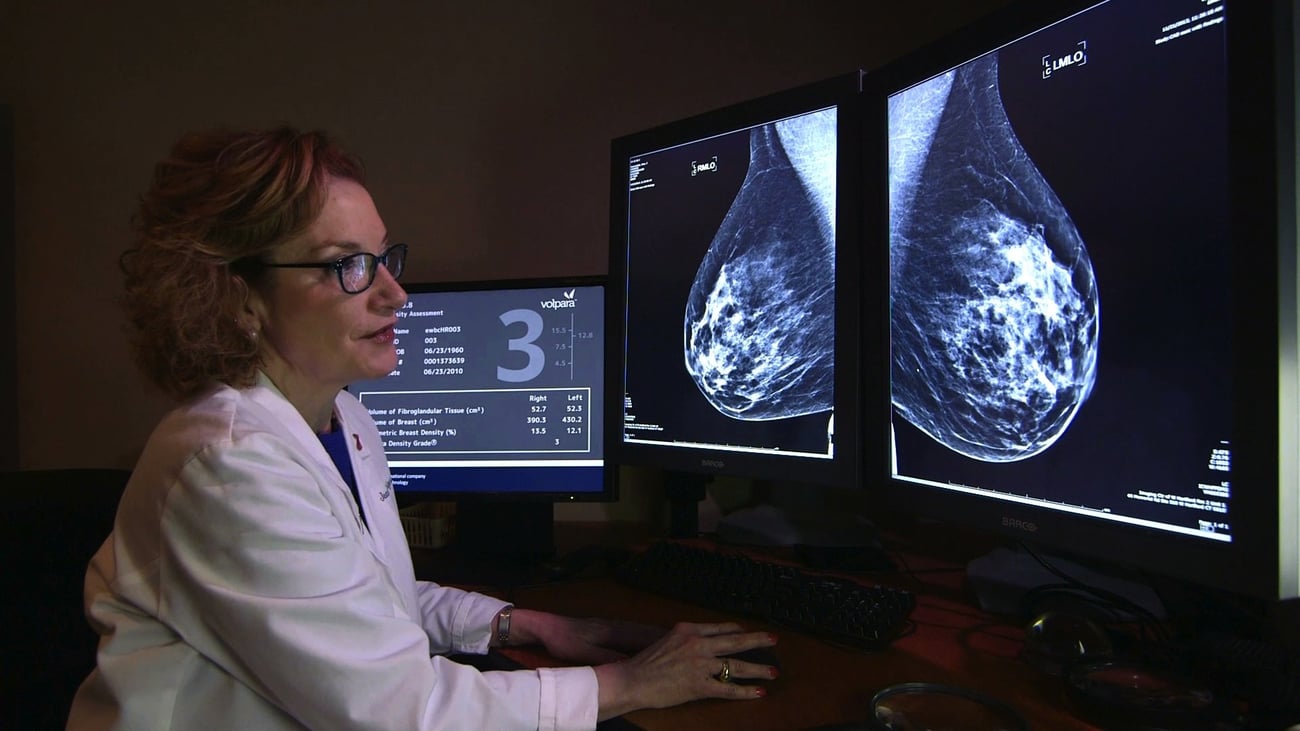
?A: The breast consists of fat and dense tissue, our software reads a breast x-ray and automatically tells the radiologist what proportion of a woman’s breast is dense tissue, something they could only guess at before.
The more dense tissue you have, the higher the risk of developing breast cancer, the higher the risk of developing an aggressive breast cancer, and the higher the risk that the cancer might not be seen on the breast x-ray by the radiologist. So, using our measurements, radiologists globally are now starting to personalise breast cancer screening protocols.? Every day now we hear stories of breast cancers being detected early due to the revolution that’s going on in breast cancer screening and it is humbling to be part of it.
Q: Your technology has been picked by University of California’s medical centres to assess at-risk breast cancer people. Tell us how you have been selling this technology, what it means to the company?
?A: The key to many of the big sales is having clinical validation published. Over the last four years, we’ve been part of numerous trials around the world, many of which are publishing now into major medical journals. So far, all the trials have been incredibly positive about our products. In California, they compared our product to an in-house product and to a direct competitor, and selected us – a very major achievement and testament to the R&D team.

(LtoR) Sir Michael Brady, founder and director of Matakina, Dr Ralph Highnam, and British High Commissioner to New Zealand, Jonathan Sinclair LVO at the company’s office, official opening. Picture sourced from Matakina’s Facebook page.
Q: Can you tell us a bit about the development of the software, what were the challenges building it, testing it, and marketing it?
?There are various angles to this:
The science component: The key science component had to be a step above whatever else was already on the market, fortunately with our scientific advisory team in Toronto, Oxford and Nijmegen (Netherlands) we had a great brains trust to provide fuel to the Wellington R&D team.
Meeting regulatory standards: We are a medical device so we have to build the product to regulatory standards around the world, notably the FDA’s Good Manufacturing Principles, and the Medical Device Directives in the EU.? Getting software engineers to build products to that standard takes time and there are not many people in NZ with FDA experience. Fortunately, Wellington has some exceptional software engineers, and with judicious hiring from abroad in key roles we’ve been able to reach a very high standard indeed. The remarkably few issues we see in the field is a great testament to the engineering and test teams – we really do it get it right, first time.
Top talent: For marketing, we’ve slowly but surely been increasing the US and global sales & marketing teams. We’ve taken the approach of focussing on the US market, and of hiring only exceptional sales & marketing people. That’s expensive, but with new products and in an industry in a state of flux, only the best is good enough.
Q: Would you be able to say what kind of sales you have had for your software? What has the growth been in 2014, over 2013; and what are the prospects going forward?
A: For FY2015, we’re going to have double product revenues versus FY2014, which itself was 50% higher than FY2013. With this GE deal, we can look ahead with confidence to FY2016 and with the validation papers now coming out we’re getting some very major deals lined up.?
Q: What are your biggest challenges creating a technology-based company out of NZ?
?A: For us, it’s been the FDA and medical imaging skills and building up that trusted sales and marketing network – initially I used to travel every few weeks, but now I can travel every 3 months due to the quality of the people that we have. Funding has not been an issue so far and we have an exceptional board and investment advisers and we closed a NZ$5.5M funding round last year.
At the conception of company, the government, through Grow Wellington, NZTE and Callaghan Innovation were great in their practical and financial support.
Some updates from CEO Ralph Highnam on 2014
Q: What are the benefits of operating out of a small country at the end of the world?
?A: Public and government support has been outstanding. ?
Q: What does success look like for you?
A: ?The number of lives saved because of our software, revenues and profitability.? We recently scanned our four-millionth woman; a significant milestone for a small company.
Q: What has been the hardest part of your journey so far? Is it raising money to fund development?
?A: The industry is in a state of flux, staying in touch and understanding the US market and getting ahead of the game has been difficult to do from NZ, we’ve achieved it and stayed ahead of the competition, but we know we need to keep innovating and believing in the core science.
Q: Some parting thoughts about operating in NZ?
?A: Funding has not been an issue, but it is still sad for us that we have most of the major research institutes around the world working with us but no significant research underway in NZ. ?NZ has the opportunity to lead the way in high quality personalised breast cancer screening, but apart from two private clinics in Auckland (St Marks and Auckland Breast Centre), we’ve seen very little public programme interest.